Abstract. Cerebrovascular diseases and COVID-19 are comorbid conditions. Endothelial dysfunction is one of the pathogenetic mechanisms for cerebrovascular diseases and COVID-19 development. Laboratory feature of endothelial dysfunction is a change in the level of endothelial dysfunction biochemical markers in the blood serum of patients.
The aim: to study the diagnostic and prognostic value of biochemical markers of endothelial dysfunction in patients with dyscirculatory encephalopathy (DE) who underwent COVID-19.
Material and methods. For the period from 03/01/22 to 05/31/22, 172 patients were examined, including 137 (79,6%) female and 35 (20.4%) male patients who had COVID-19 and are being examined at the base polyclinic No. 2 of the Central clinical medical and sanitary unit named after honored doctor of Russia V.A. Egorov, Ulyanovsk. Median time from the onset of COVID-19 to examination was 4.8 months. DE was not found in 6% of patients who underwent COVID-19, stage I DE was present in 45%, stage II in 27%, stage III in 22% of participants of the study. Blood sampling was carried out once during the all examination period. Levels of vasculoendothelial growth factor (VEGFA), interleukins 6, 10, 18, tumor necrosis factor-alpha, and monocytic chemotactic protein 1 were studied in blood serum. The Mann–Whitney U-test was used to test the hypothesis of a difference in the samples of patient groups. For all types of statistical analysis, differences were considered to be significant at the achieved significance level p <0,05.
Results. According to our research, with an increase of the age of patients who have undergone COVID-19, and the DE stage the level of VEGFA in serum was also increasing (p <0,05).
Conclusion. From studied cytokines, the predictive role as a marker of endothelial dysfunction was shown by VEGFA. Its high level in blood serum is associated with the age of DE patients, who have undergone COVID-19, and with the stage of DE they had.
ВВЕДЕНИЕ
Цереброваскулярные заболевания и COVID-19 являются коморбидными состояниями. Кроме того, COVID-19 выступает значимым фактором риска возникновения цереброваскулярных осложнений [1–3]. Вирус SARS-CoV-2 приводит как к прямому, так и опосредованному поражению центральной нервной системы (ЦНС) [4–6].
Один из патогенетических механизмов развития цереброваскулярных заболеваний и COVID-19 – эндотелиальная дисфункция [6–11]. Лабораторным отображением эндотелиальной дисфункции служит изменение уровня биохимических маркеров дисфункции эндотелия в сыворотке крови пациентов. Наиболее показательны такие биохимические маркеры дисфункции эндотелия, как васкулоэндотелиальный фактор роста (VEGFA), интерлейкин 6 (IL-6), IL-10, IL-18, фактор некроза опухоли-альфа (TNF-α), моноцитарный хемотаксический белок 1 (MCP-1).
Новая коронавирусная инфекция, вызванная вирусом SARS-CoV-2, характеризуется системной гипервоспалительной реакцией с выраженным повышением содержания провоспалительных цитокинов, которая получила название «цитокиновый шторм». У пациентов с COVID-19 повышен уровень циркулирующих IL-6, IL-10, IL-18, MCP-1, TNF-α и VEGF. Повышение содержания провоспалительных цитокинов отражает активность, тяжесть течения патологического процесса, коррелирует с неблагоприятным прогнозом [10, 12–27].
При хронической ишемии головного мозга происходят неинфекционные процессы воспаления в головном мозге, обусловленные ишемией; при этом роль цитокинов в патогенезе велика [28].
Цель нашего исследования – изучение диагностического и прогностического значения биохимических маркеров эндотелиальной дисфункции у пациентов с дисциркуляторной энцефалопатией (ДЭ), перенесших COVID-19.
МАТЕРИАЛ И МЕТОДЫ
За период с 01.03.2022 по 31.05.2022 было обследовано 172 пациента, включая 137 (79,6%) женщин и 35 (20,4%) мужчин, перенесших COVID- 19, которые проходят углубленное диспансерное обследование на базе поликлиники № 2 ГУЗ «Центральная клиническая медико-санитарная часть им. заслуженного врача России В.А. Егорова» г. Ульяновска в рамках клинического исследования «Постковидный синдром: соматические, неврологические, возрастные особенности течения и реабилитации». По данным нашего исследования, возраст обследованных женщин составлял от 18 до 88 лет (средний возраст 58,0±16 лет), мужчин – от 22 до 81 года (средний возраст 54,0±17 лет). Средний возраст всех пациентов составил 57,0±15 лет.
Исследование одобрено локальным этическим комитетом института медицины, экологии и физической культуры Ульяновского государственного университета (протокол от 15.01.2022 № 1). От пациентов было получено добровольное информированное согласие на участие в исследование.
В исследование были включены пациенты с лабораторно подтвержденным COVID-19 (шифр по МКБ-10 U 07.1), перенесшие это заболевание от 3 мес до 1 года до начала исследования. Среднее время от момента заболевания COVID-19 до осмотра составило 4,8 мес.
Критерием невключения было наличие в анамнезе у пациента системных, аутоиммунных и онкологических заболеваний.
Всем пациентам выполнялось полное клинико-неврологическое исследование. При оценке неврологического статуса пациентов с ДЭ выделялся ведущий неврологический синдром в соответствии с классификацией Научного центра неврологии (2001). На каждого пациента была оформлена унифицированная карта.
Большинство обследованных больных (78,5%) с диагнозом COVID-19 получало лечение амбулаторно.
Артериальная гипертензия (АГ) была зарегистрирована у 75% пациентов, перенесших COVID-19, при этом у большинства больных (56,5%) была III стадия, 3-я степень АГ. Фибрилляция предсердий была выявлена у 4,1% обследованных пациентов, перенесших COVID-19. 42,9% участников принимали антикоагулянты (апиксабан). Сахарный диабет наблюдался у 8,7% человек, перенесших COVID-19; у 46,7% таких пациентов имела место декомпенсация заболевания на фоне COVID-19.
ДЭ не была выявлена у 6% больных, перенесших COVID-19. I стадия ДЭ имела место у 45%, II стадия – у 27%, III стадия – у 22% пациентов, перенесших COVID-19.
Забор крови был проведен однократно в период обследования. В сыворотке крови исследовались следующие цитокины: VEGFA, IL-6, IL-10, IL-18, TNF-α, MCP-1. Использовались наборы для иммуноферментного анализа производства «Вектор Бест» (г. Новосибирск, Россия). В качестве контрольных значений использовались данные, предложенные в протоколе производителя.
Статистическая обработка данных осуществлялась с применением пакета прикладных программ Microsoft Excel 2010 и StatTech v. 2.8.8 (разработчик – ООО «Статтех», Россия). Для проверки гипотезы о различии выборок групп больных использовался U-критерий Манна–Уитни. Для всех видов статистического анализа различия считались достоверными при достигнутом уровне значимости p <0,05.
РЕЗУЛЬТАТЫ И ОБСУЖДЕНИЕ
По данным литературы, к наиболее частым симптомам long-COVID относятся повышенная утомляемость, головная боль, потеря вкуса и/или обоняния, миалгии, одышка и головокружение [29–32].
Основные жалобы исследованных пациентов, перенесших COVID-19, представлены в таблице 1.

Возможные причины long-COVID включают остаточные повреждения, сохраняющиеся после острой фазы коронавирусной инфекции, персистенцию вируса в организме, пролонгирование системного иммунного воспаления и ухудшение течения сопутствующих заболеваний [33].
Нами оценивались уровни цитокинов в сыворотке крови у пациентов в зависимости от времени, прошедшего от перенесенного COVID-19 до осмотра: через 3–4, 5–6 и более 6 мес. Полученные данные свидетельствуют о снижении уровня провоспалительных цитокинов (VEGFA, IL-6, IL-18, MCP-1) в сыворотке крови пациентов: максимальный уровень этих биомаркеров был выявлен у пациентов через 3–4 мес, минимальный – более чем через 6 мес после COVID-19. Уменьшение уровня провоспалительных цитокинов (VEGFA, IL-6, IL-18, MCP-1) в сыворотке крови, вероятно, обусловлено уменьшением со временем выраженности воспалительного ответа на вирус SARS-CoV-2. Однако статистически значимых различий между обследованными временными группами (3–4, 5–6 и более 6 мес после COVID-19) обнаружено не было (p >0,05), что может свидетельствовать о пролонгировании системного иммунного воспаления (рис. 1).
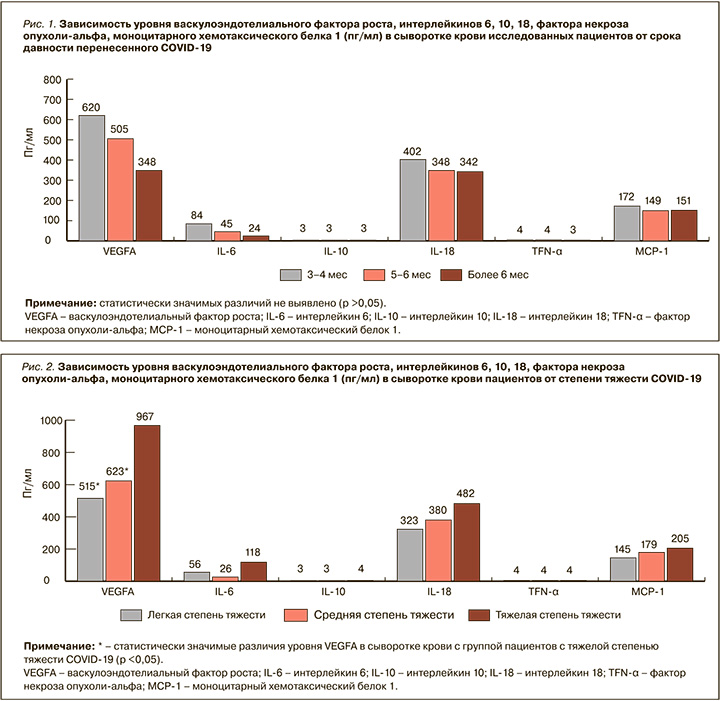
Данные нашего исследования свидетельствуют, что в остром периоде COVID-19 легкая степень тяжести заболевания наблюдалась у 75,6%, средняя – у 12,8%, тяжелая – у 11,6% пациентов. Степень тяжести течения острого периода COVID- 19 определялась в соответствии временными методическими рекомендациями Минздрава России (версия 15 от 22.02.2022).
Нами выявлено нарастание уровня таких провоспалительных цитокинов, как VEGFA, IL-6, IL-18, MCP-1, в сыворотке крови пациентов, перенесших COVID-19, в зависимости от тяжести течения острого периода новой коронавирусной инфекции. Отмечено, что уровень этих цитокинов меньше у пациентов с легкой степенью тяжести COVID-19 и выше у больных с тяжелой степенью. Однако статистически значимое увеличение касалось только VEGFA (p <0,05; рис. 2). По данным нашего исследования, так же как и по литературным данным, выраженность воспалительной реакции с высвобождением большого количества провоспалительных цитокинов прямо коррелирует с объемом повреждения легких, острым респираторным дистресс-синдромом, полиорганной недостаточностью и неблагоприятным прогнозом [34, 10, 13].
По данным нашего исследования, с увеличением возраста пациентов, перенесших COVID- 19, нарастал уровень VEGFA в сыворотке крови (p <0,05; рис. 3). Возможно, это связано с тем, что с возрастом повышается степень выраженности дисциркуляторных изменений, приводящих к большей стимуляции ангиогенеза посредством экспрессии VEGFA.
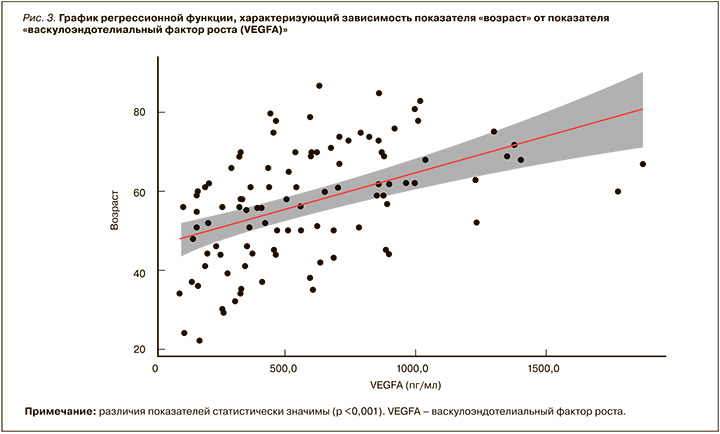
Увеличение уровня VEGFA в сыворотке крови пациентов, перенесших COVID-19, в зависимости от степени АГ оказалось статистически незначимо (p >0,05; рис. 4). Таким образом, можно предположить, что неоангиогенез характерен для постковидного синдрома, вне зависимости от степени АГ.
По данным нашего исследования, с увеличением стадии ДЭ нарастает уровень VEGFA в сыворотке крови пациентов, перенесших COVID-19 (p >0,05). При этом статистически значимые различия получены по уровню VEGFA в сыворотке крови у пациентов со II и III стадией ДЭ в сравнении с группой пациентов без этой патологии и с I стадией заболевания (p <0,05; рис. 5). Вероятно, это связано с тем, что ангиогенез выступает одним из главных патогенетических механизмов процесса адаптации вещества головного мозга к гипоксии. В зоне ишемии компенсаторно возникает индукция ангиогенеза путем экспрессии VEGFA при ДЭ [35].
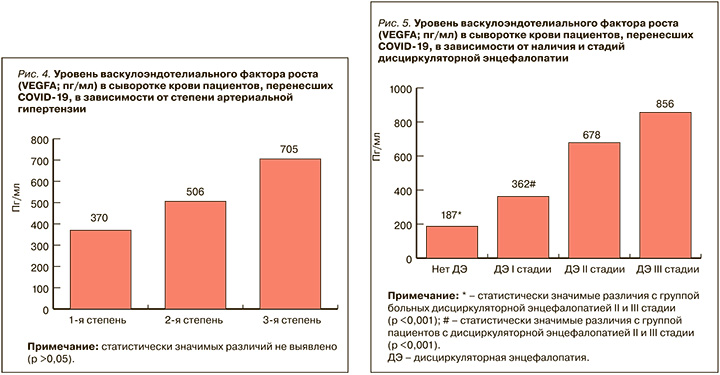
При прогрессировании ДЭ нарастают гипоксия вещества головного мозга, выраженность мелкоочаговых и диффузных изменений белого вещества головного мозга. Это ведет к увеличению уровня VEGFA с нарастанием стадий ДЭ, что, в свою очередь, свидетельствует о повышении активности ангиогенеза и процессов, противодействующих атеросклеротическому повреждению сосудистой стенки, усиливающих формирование эндотелиальных клеток и предотвращающих десквамацию эндотелия.
Выраженное изменение уровня VEGFA в сыворотке крови пациентов с ДЭ, перенесших COVID- 19, предполагает его патогенетическую роль как в развитии воспалительной реакции, так и поддержании эндотелиальной дисфункции, модулированных гипоксическим ответом клеток на вирусную инфекцию SARS-CoV-2.
По литературным данным, в плазме крови пациентов с ДЭ выявлено значительное повышение концентрации цитокинов IL-6 и IL-18 [36], что, возможно, обусловлено нарастанием ишемии и степени выраженности эндотелиальной дисфункции с увеличением стадии ДЭ. Так, при бессимптомной церебральной или миокардиальной ишемии и гипоксии повышается уровень IL-6 [37]. Кроме того, возрастание уровня IL-6 в сыворотке крови ассоциировано с развитием ишемической болезни сердца, острых нарушений мозгового кровообращения и смертью от сердечно-сосудистых заболеваний [38, 39].
По данным нашего исследования, уровень IL-6 в сыворотке крови пациентов, перенесших COVID- 19, в случае ДЭ III стадии выше, чем при I стадии этого заболевания (p <0,05). Уровень IL18 в сыворотке крови пациентов, перенесших COVID-19, выше у больных ДЭ I, II, III стадий, чем у лиц без этой патологии (p <0,05), при отсутствии различий между стадиями ДЭ (табл. 2).
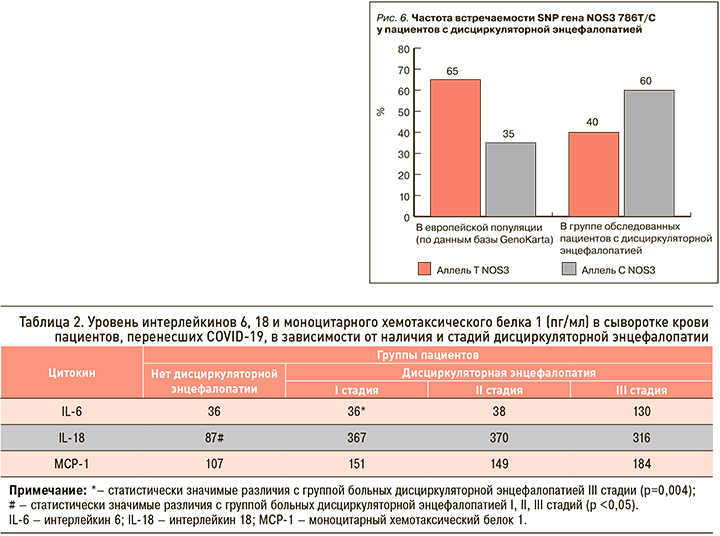
Одним из медиаторов эндотелия служит NO, который приводит к расширению сосудов, снижению артериального давления, ингибированию агрегации тромбоцитов и окисления липопротеинов низкой плотности. Эндотелиальная NO-синтаза – один из ферментов группы NOS, которые катализируют синтез оксида азота [40]. Эндотелиальная NO-синтаза кодируется геном NOS3. Описан ряд полиморфизмов этого гена [41, 42]. Основной мутацией, способной влиять на работу сердечно-сосудистой системы, является SNP 786T/C. Аллель С в промоторной части гена связана с низкой продукцией белка, который блокирует экспрессию NOS3 и уменьшает вазодилатацию [43, 44]. Этот генный вариант ассоциирован с АГ, острым инфарктом миокарда, спазмом коронарной артерии, ишемическим инсультом, повышенным риском атеросклероза [45, 46].
В результате нашего исследования установлено, что частота встречаемости SNP гена NOS3 786T/C была в 2 раза выше в обследованной группе пациентов с ДЭ по сравнению с европейской популяцией (рис. 6).
В норме в эндотелии сосудов постоянно происходит высвобождение NO, который оказывает вазодилатирующее, антиагрегантное и антипролиферативное действие. Нарушение метаболизма NO играет важную роль в патогенезе эндотелиальной дисфункции. Наличие SNP гена NOS3 786T/C связана с низкой продукцией белка, который блокирует экспрессию NOS3. Повышенная встречаемость SNP гена NOS3 786T/C в группе пациентов с ДЭ обусловлена их генетической предрасположенностью к более выраженной эндотелиальной дисфункции.
ЗАКЛЮЧЕНИЕ
1. Основными жалобами пациентов, перенесших COVID-19, в нашем исследовании были общая слабость (86,6%), боли в суставах и мышцах (62,2%), нарушение обоняния (61,0%), нарушение вкуса (55,8%). У большей части обследованных пациентов отягощен анамнез по сердечно-сосудистым заболеваниям (у 81,4%).
2. Выявлено уменьшение уровня провоспалительных цитокинов (VEGFA, IL-6, IL-18, MCP-1) в сыворотке крови пациентов, перенесших COVID-19, по мере удаления от острого периода заболевания.
3. Чем тяжелее протекал острый период COVID- 19, тем выше уровень VEGFA в сыворотке крови пациентов на момент осмотра (p <0,05), что, вероятно, связано с компенсаторно возникающей стимуляцией ангиогенеза при ДЭ путем экспрессии VEGFA.
4. Среди изученных цитокинов прогностическую роль в качестве маркера эндотелиальной дисфункции продемонстрировал VEGFA. Его высокий уровень в сыворотке крови ассоциирован с возрастом больных ДЭ, перенесших COVID-19, и стадией ДЭ у них.
5. Выраженность эндотелиальной дисфункции ассоциирована с повышенной встречаемостью SNP гена NOS3 786T/C в группе пациентов с ДЭ, что может говорить о генетической предрасположенности к ней у этой категории пациентов.
1. Inciardi R.M Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020; 5(7): 819–24. https://dx.doi.org/10.1001/jamacardio.2020.1096.
2. Huang C., Wang Y., Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395(10223): 497–506. https://dx.doi.org/10.1016/S0140-6736(20)30183-5.
3. Mao L., Jin H., Wang M. et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77(6): 683–90. https://dx.doi.org/10.1001/jamaneurol.2020.1127.
4. Wang D., Hu B., Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323(11): 1061–69. https://dx.doi.org/10.1001/jama.2020.1585. Erratum in: JAMA. 2021; 325(11): 1113.
5. Garrigues E., Janvier P., Kherabi Y. et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020; 81(6): e4–e6. https://dx.doi.org/10.1016/j.jinf.2020.08.029.
6. Bourgonje A.R., Abdulle A.E., Timens W. et al. Angiotensinconverting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020; 251(3): 228–48. https://dx.doi.org/10.1002/path.5471.
7. Barrantes F.J. Central nervous system targets and routes for SARS-CoV-2: Current views and new hypotheses. ACS Chem Neurosci. 2020; 11(18): 2793–803. https://dx.doi.org/10.1021/acschemneuro.0c00434.
8. Brann D.H., Tsukahara T., Weinreb C. et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. SciAdv. 2020; 6(31): eabc5801. https://dx.doi.org/10.1126/sciadv.abc5801.
9. Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020; 16(11): 636–44. https://dx.doi.org/10.1038/s41582-020-0398-3.
10. Jose R.J., Manuel A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir Med. 2020; 8(6): e46–47. https://dx.doi.org/10.1016/S2213-2600(20)30216-2.
11. Верткин А.Л., Авдеев С.Н., Ройтман Е.В. с соавт. Вопросы лечения COVID-19 с позиции коррекции эндотелиопатии и профилактики тромботических осложнений. Согласованная позиция экспертов. Профилактическая медицина. 2021; 24(4): 45–51. [Vertkin A.L., Avdeev S.N., Roitman E.V. et al. Questions of COVID-19 treatment from the position of correction of endotheliopathy and prevention of thrombotic complications. Coordinated position of experts. Profilakticheskaya meditsina = Preventive Medicine. 2021; 24(4): 45–51 (In Russ.)]. https://dx.doi.org/10.17116/profmed20212404145. EDN: MYILVR.
12. Mehta P., McAuley D.F., Brown M. et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020; 395(10229): 1033–34. https://dx.doi.org/10.1016/S0140-6736(20)30628-0.
13. Бобкова С.С., Жуков А.А., Проценко Д.Н. с соавт. Критический анализ концепции «цитокиновой бури» у пациентов с новой коронавирусной инфекцией COVID-19. Обзор литературы. Вестник интенсивной терапии им. А.И. Салтанова. 2021; (1): 57–68. [Bobkova S.S., Zhukov A.A., Protsenko D.N. et al. Critical analysis of «cytokine storm» concept in patients with new coronavirus infection COVID-19. Literature review. Vestnik intensivnoy terapii imeni A.I. Saltanova = A.I. Saltanov Bulletin of Intensive Care. 2021; (1): 57–68 (In Russ.)]. https://dx.doi.org/10.21320/1818-474X-2021-1-57-68. EDN: APFKIT.
14. Liu Y., Zhang C., Huang F. et al. Elevated plasma level of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl Sci Rev. 2020; 7(6): 1003–11. https://dx.doi.org/10.1093/nsr/nwaa037.
15. Liu Jing, Li S., Liu Jia et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020; 55: 102763. https://dx.doi.org/10.1016/j.ebiom.2020.102763.
16. Al-Faraj H.A.M.H., Al-Hasnawi A.T.N., Al-Mamori M.A.A. Elevated serum levels of MCP-1 and IP-10 chemokines in patients with COVID-19 infection. NeuroQuantology. 2022; 20(6): 6769–79.
17. Гришаева А.А., Понежева Ж.Б., Чанышев М.Д. с соавт. Состояние цитокиновой системы у больных с тяжелой формой COVID- 19. Лечащий врач. 2021; (6): 48–51. [Grishaeva A.A., Ponezheva Zh.B., Chanyshev M.D. et al. The state of the cytokine system in patients with severe COVID-19. Lechashchiy vrach = Attending Physician. 2021; (6): 48–51 (In Russ.)]. https://dx.doi.org/10.51793/OS.2021.24.6.010. EDN: QXGEAQ.
18. Chen Y., Wang J., Liu C. et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med. 2020; 26(1): 97. https://dx.doi.org/10.1186/s10020-020-00230-x.
19. Han H., Ma Q., Li C. et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020; 9(1): 1123–30. https://dx.doi.org/10.1080/22221751.2020.1770129.
20. Del Valle D.M., KimSchulze S., Huang H. et al. An inflammatory cytokine signature pre-dicts COVID19 severity and survival. Nat Med. 2020; 26(10): 1636–43. https://dx.doi.org/10.1038/s41591-020-1051-9.
21. Liu Y., Chen D., Hou J. et al. An intercorrelated cytokine network identified at the center of cytokine storm predicted COVID-19 prognosis. Cytokine. 2021; 138: 155365. https://dx.doi.org/10.1016/j.cyto.2020.155365.
22. Chen G., Wu D.I., Guo W. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130(5): 2620–29. https://dx.doi.org/10.1172/JCI137244.
23. Herold T., Jurinovic V., Arnreich C. et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020; 146(1): 128–36.e4. https://dx.doi.org/10.1016/j.jaci.2020.05.008.
24. Ruan Q., Yang K., Wang W. et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46(5): 846–48. https://dx.doi.org/10.1007/s00134-020-05991.
25. Chen G., Wu D., Guo W. et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130(5): 2620–29. https://dx.doi.org/10.1172/JCI137244.
26. Zhu Z., Cai T., Fan L. et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020; 95:332–39. https://dx.doi.org/10.1016/j.ijid.2020.04.041
27. Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020; 189(3): 428–37. https://dx.doi.org/10.1111/bjh.16659.
28. Popa C., Netea M.G., van Riel P.L.C.M. et al. The role of TNF-α in chronic inflammato-ry conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 2007; 48(4): 751–62. https://dx.doi.org/10.1194/jlr.R600021-JLR200.
29. Долгополов И.С., Менткевич Г.Л., Рыков М.Ю., Чичановская Л.В. Неврологические нарушения у пациентов с long COVID синдромом и методы клеточной терапии для их коррекции: обзор литературы. Сеченовский вестник. 2021; 12(3): 56–67. [Dolgopolov I.S., Mentkevich G.L., Rykov M.Y., Chichanovskaya L.V. Neurological disorders in patients with long COVID syndrome and methods of cell therapy for their correction: literature review. Sechenovskiy vestnik = Bulletin of Sechenov University. 2021; 12(3): 56–67 (In Russ.)]. https://dx.doi.org/10.47093/2218-7332.2021.12.3.56-67. EDN: LDAZRR.
30. Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77(6): 683–90. https://dx.doi.org/10.1001/jamaneurol.2020.1127.
31. Guan W.J., Ni Z.Y., Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18): 1708–20. https://dx.doi.org/10.1056/NEJMoa2002032.
32. Carfi A., Bernabei R., Landi F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020; 324(6): 603–5. https://dx.doi.org/10.1001/jama.2020.12603.
33. Nath A. Long-Haul COVID. Neurology. 2020; 95(13): 559–60. https://dx.doi.org/10.1212/WNL.0000000000010640.
34. Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020; 181(5): 1036–45.e9. https://dx.doi.org/10.1016/j.cell.2020.04.026
35. Beck H., Plate K.H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009; 117(5): 481–96. https://dx.doi.org/10.1007/s00401-009-0483-6.
36. Конопля А.И., Ласков В.Б., Шульгинова А.А. Иммунные и оксидантные нарушения у больных с хронической ишемией мозга и их коррекция. Журнал неврологии и психиатрии им. C.C. Корсакова. 2015; 115(11): 28–32. [Konoplya A.I., Laskov V.B., Shulginova A.A. Immune and oxidative disorders in patients with chronic brain ischemia and their correction. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova = S.S. Korsakov Journal of Neurology and Psychiatry. 2015; 115(11): 28–32 (In Russ.)]. https://dx.doi.org/10.17116/jnevro201511511128-32. EDN: VHCXBP.
37. Shinohara T., Takahashi N., Okada N. et al. Interleukin-6 as an independent predictor of future cardiovascular events in patients with type-2 diabetes without structural heart disease. J Clin Exp Cardiology. 2012; 3(9): 209. https://dx.doi.org/10.4172/2155-9880.1000209.
38. Danesh J., Kaptoge S., Mann A.G. et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 2008; 5(4): 78. https://dx.doi.org/10.1371/journal.pmed.0050078.
39. Tehrani D.M., Gardin J.M., Yanez D. et al. Impact of inflammatory biomarkers on relation of high density lipoproteincholesterol with incident coronary heart disease: Cardiovascular health study. Atherosclerosis. 2013; 231(2): 246–51. https://dx.doi.org/10.1016/j.atherosclerosis.2013.08.036.
40. Березовская Г.А., Ганюков В.И., Карпенко М.А. Рестеноз и тромбоз внутри стента: патогенетические механизмы развития и прогностические маркеры. Российский кардиологический журнал. 2012; 17(6): 91–95. [Berezovskaya G. A., Ganyukov V.I., Karpenko M.A. Restenosis and thrombosis inside the stent: pathogenetic mechanisms of development and prognostic markers. Rossiyskiy kardiologicheskiy zhurnal = Russian Journal of Cardiology. 2012; 17(6): 91–95 (In Russ.)]. https://dx.doi.org/10.15829/1560-4071-2012-6-91-95. EDN: PJOIDF.
41. Naber C.K., Frey U.H., Oldenburg O. et al. Relevance of the NOS3 T-786C and Glu298Asp variants in the endothelial nitric oxide synthase gene for cholinergic and adrenergic coronary vasomotore responses in man. Basic Res Cardiol. 2005; 100(5): 453–60. https://dx.doi.org/10.1007/s00395-005-0530-y.
42. Куба А.А., Никонова Ю.М., Феликсова О.М. с соавт. Ассоциация генетического полиморфизма гена эндотелиальной синтазы оксида азота с сердечно-сосудистой патологией. Современные проблемы науки и образования. 2015; (3): 19. [Kuba A.A., Nikonova N.M., Feliksova O.M. et al. Association of genetic polymorphism of endothelial nitric oxide synthase gene with cardio-vascular pathology. Sovremennye problemy nauki i obrazovaniya = Modern Problems of Science and Education. 2015; (3): 19 (In Russ.)]. EDN: TYSGSJ.
43. Dosenko V.E., Zagoriy V.Y., Haytovich N.V. et al. Allelic polymorphism of endothelial NO-synthase gene and its functional manifestations. Acta Biochim Pol. 2006; 53(2): 299–302.
44. Yaghoubi A.R., Khaki-Khatibi F. T-786C single-nucleotide polymorphism (SNP) of endothelial nitric oxide synthase gene and serum level of vascular endothelial relaxant factor (VERF) in nondiabetic patients with coronary artery disease. African J Biotechnol. 2012; 11(93): 15945–49.
45. Cruz-Gonzalez I., Corra E., Sanchez-Ledesma M. et al. Association between -T786C NOS3 polymorphism and resistant hypertension: A prospective cohort study. BMC Cardiovasc Disord. 2009; 9: 35. https://dx.doi.org/10.1186/1471-2261-9-35.
46. Страмбовская Н.Н., Витковский Ю.А., Смоляков Ю.Н. с соавт. Ишемический инсульт – заболевание с высокой степенью генетической предрасположенности. Забайкальский медицинский вестник. 2019; (1): 91–101. [Strambovskaya N.N., Vitkovsky Yu.A., Smolyakov Yu.N. et al. Ischemic stroke – a disease with a high degree of genetic predisposition. Zabaykal’skiy meditsinskiy vestnik = Transbaikal Medical Bulletin. 2019; (1): 91–101 (In Russ.)]. https://dx.doi.org/10.52485/19986173_2019_1_91. EDN: AXSBMR.
Viktor V. Mashin, MD, professor, head of the Department of neurology, neurosurgery and medical rehabilitation, Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
. ORCID: https://orcid.org/0000-0003-0085-3727
Dinara R. Dolgova, PhD in Biological Sciences, associate professor of the Department of physiology and pathophysiology, director of Scientific research physicobiological center, Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
. ORCID: https://orcid.org/0000-0001-5475-7031
Lyudmila A. Belova, MD, professor, dean of the Faculty of medicine of the Institute of medicine, ecology and physical culture, Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
. ORCID: https://orcid.org/0000-0002-9585-5604
Elena Yu. Kotova, PhD in Medical Sciences, associate professor of the Department of neurology, neurosurgery and medical rehabilitation, Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
. ORCID: https://orcid.org/0009-0004-2293-3183
Landysh R. Kruglova, postgraduate student of the Department of neurology, neurosurgery and medical rehabilitation, Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
. ORCID: https://orcid.org/0009-0008-9665-6915
Anastasia P. Statenina, student of the Faculty of medicine named after T.Z. Biktimirov of the Institute of medicine, ecology and physical culture (group LD-O-19/3), Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
Andrey A. Kozin, student of the Faculty of medicine named after T.Z. Biktimirov of the Institute of medicine, ecology and physical culture (group LD-O-17/6), Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
Rumiya R. Israfilova, student of the Faculty of medicine named after T.Z. Biktimirov of the Institute of medicine, ecology and physical culture (group LD-O-18/9), Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:
Daria K. Martynova, student of the Faculty of medicine named after T.Z. Biktimirov of the Institute of medicine, ecology and physical culture(group LD-O-18/11), Ulyanovsk State University. Address: 4321017, Ulyanovsk, 42 L. Tolstogo Str. E-mail:








